
THE MYCOPLASMA GROUP AT CIRAD-ASTRE
UMR ASTRE AnimalS – health – Territories – Risks – Ecosystems
The mycoplasma group is part of ASTRE (AnimalS, health, Territories, Risks, Ecosystems), a pluri-disciplinary research unit of CIRAD and INRA focused on the improvement of animal health, public health and food security in developing countries.
Control of ruminant mycoplasmoses
Ruminant mycoplasmoses are responsible for serious losses in livestock production all over the world. The mycoplasma group at CIRAD-ASTRE, is dedicated to the study of ruminant mycoplasmoses, such as Contagious Bovine Pleuropneumonia and Contagious Caprine Pleuropneumonia, having a great economic impact in developing countries and threatening free territories, notably in Europe.
Objectives and main research activities
To understand the diversity and evolution of mycoplasma genomes
We compare the genomes of mycoplasma strains at local to world-wide scales for classification and diagnostic purposes, for epidemiological investigations and to study the origin and evolution of these pathogens (their emergence and dissemination in different countries and continents).
Suggested reading
Dupuy V, Verdier A, Thiaucourt F, Manso-Silván L (2015). A large-scale genomic approach affords unprecedented resolution for the molecular epidemiology and evolutionary history of contagious caprine pleuropneumonia. Veterinary Research;46:74. (https://doi.org/10.1186/s13567-015-0208-x)
To understand the mechanisms involved in pathogenesis
We develop and apply diverse approaches to unravel the mechanisms involved in mycoplasma virulence and attenuation and to characterise the immune response of the host upon infection, which is essential for the development of novel control strategies.
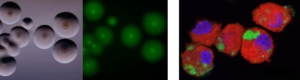
Suggested reading
Bonnefois T, Vernerey MS, Rodrigues V, Totté P, Puech C, Ripoll C, Thiaucourt F, Manso-Silván L (2016). Development of fluorescence expression tools to study host-mycoplasma interactions and validation in two distant mycoplasma clades. J Biotechnol. 2016 Oct 20;236:35-44. (https://doi.org/10.1016/j.jbiotec.2016.08.006)
Rodrigues V, Holzmuller P, Puech C, Wesonga H, Thiaucourt F, Manso-Silván L (2015). Whole Blood Transcriptome Analysis of Mycoplasma mycoides subsp. mycoides-Infected Cattle Confirms Immunosuppression but Does Not Reflect Local Inflammation. PLoS One 2015 Oct 2;10(10):e0139678. (https://doi.org/10.1371/journal.pone.0139678)
Totté P, Puech C, Rodrigues V, Bertin C, Manso-Silván L, Thiaucourt F (2015). Free exopolysaccharide from Mycoplasma mycoides subsp. mycoides possesses anti-inflammatory properties. Veterinary Research; 4622 (21 October 2015). (https://doi.org/10.1186/s13567-015-0252-6)
Bertin C, Pau-Roblot C, Courtois J, Manso-Silván L, Tardy F, Poumarat F, Citti C, Sirand-Pugnet P, Gaurivaud P, Thiaucourt F (2015). Highly Dynamic Genomic Loci Drive the Synthesis of Two Types of Capsular or Secreted Polysaccharides within the Mycoplasma mycoides Cluster. Applied and Environmental Microbiology 81, 676-687. (https://doi.org/10.1128/AEM.02892-14)
To develop and validate performant diagnostic assays for disease surveillance and control
We develop molecular detection and typing tools (based on the analysis of genomic polymorphisms), as well as specific sero-diagnostic assays, which have been extensively validated in the field. A better understanding of the distribution and prevalence of pathogens is essential for the implementation of appropriate control strategies.
Suggested reading
Peyraud A, Poumarat F, Tardy F, Manso-Silván L, et al., Thiaucourt F. (2014). An international collaborative study to determine the prevalence of contagious caprine pleuropneumonia by monoclonal antibody-based cELISA. BMC Vet Res;10:48. (https://doi.org/10.1186/1746-6148-10-48)
To validate new vaccines and vaccine strategies
We characterise the protective immune response induced by vaccination for the assessment of new vaccines and vaccine formulations that we develop and validate. Our group currently leads the project MultiVacc, which aims to produce a bivalent vaccine against heartwater and contagious caprine pleuropneumonia.
Suggested reading
Totté P, Yaya A, Sery A, Wesonga H, Wade A, Naessens J, Niang M, Thiaucourt F (2013). Characterization of anamnestic T-cell responses induced by conventional vaccines against contagious bovine pleuropneumonia. Plos One. 2013;8(2):e57509. (https://doi.org/10.1371/journal.pone.0057509)
Thiaucourt F, Pible O, Miotello, G, Nwankpa N, Armengaud J (2018). Improving quality control of Contagious Caprine Pleuropneumonia vaccine with tandem mass spectrometry, Proteomics. 2018 Jun 17:e1800088. (https://doi.org/10.1002/pmic.201800088)
CIRAD-ASTRE is a reference laboratory for CBPP and CCPP for the OIE (F. Thiaucourt, OIE expert) and for the FAO
We provide reference material, expert analyses and advice for disease surveillance and control. We organise practical trainings and inter-laboratory proficiency testing for capacity building in the affected regions. CIRAD-ASTRE is accredited, ISO 17025 by the French Committee of Accreditation to perform competition ELISAs for CBPP and CCPP (http://www.cofrac.fr/; ACCRÉDITATION N° 1-2207)

World Organisation for Animal Health (OIE) reference laboratory for Contagious Bovine Pleuropneumonia and Contagious Caprine Pleuropneumonia
 Food and Agriculture Organization of the United Nations (FAO) reference centre for Contagious Bovine Pleuropneumonia, Contagious Caprine Pleuropneumonia, and other mycoplasmoses of ruminants
Food and Agriculture Organization of the United Nations (FAO) reference centre for Contagious Bovine Pleuropneumonia, Contagious Caprine Pleuropneumonia, and other mycoplasmoses of ruminants
Lab members
PhD students
Sarah GANTER
Past members
Sophie LORENZON
Contact
lucia.manso-silvan@cirad.fr
Address
CIRAD-INRA ASTRE
TA A-117/E
Campus International de Baillarguet
34398 Montpellier Cedex 5
France

